SUTURE, SPIRAL PDO 1-0 36CM 48MM 1/2″ CIR (12/BX)
| Item Id | 831251 |
| Manufacturer # | SXPD2B405 |
| Brand | STRATAFIX™ Bidirectional Knotless Tissue Control Device |
| Manufacturer | J & J Healthcare Systems |
| Country of Origin | Unknown |
| Application | Absorbable Suture with Needle |
| Coating | Uncoated |
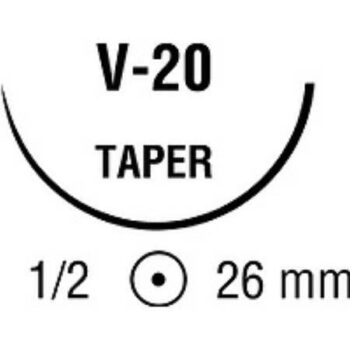
| Needle 1 Code | CTX |
| Needle 2 Code | CTX |
| Needle Length | 48 mm |
| Needle Length Range | 40.1 to 60 mm |
| Needle Material | Stainless Steel Needle |
| Needle Shape | 1/2 Circle |
| Needle Type | Taper Point Needle |
| Number of Needles | Double-Armed |
| Suture Color | Violet |
| Suture Length | 36 cm |
| Suture Length Range | 10.1 to 20 in |
| Suture Material | Spiral PDO |
| Suture Material Range | Dyed Synthetic |
| Suture Size | Size 1 |
| UNSPSC Code | 42312201 |
Features
- With significantly more points of fixation than traditional sutures, STRATAFIX™ devices give surgeons more consistent control over every pass and combine the strength and security of interrupted closure with more efficiency than continuous closure
- STRATAFIX™ Spiral PDO Knotless Tissue Control Device consists of barbed suture material, armed with a surgical needle on each end
- Barbs allow for tissue approximation without the need to tie surgical knots
- STRATAFIX™ Spiral PDO Device is comprised of dyed (violet) polyester, poly (p-dioxanone)
- The strength of the Stratafix device can be compared to USP knot strength of non-barbed sutures
- Absorption of STRATAFIX PDO Device is minimal until about 120 days, and is essentially complete within 180 days (six months)
- STRATAFIX™ Spiral PDO Device contains bidirectionally oriented barbs to anchor tissues and does not require knots to approximate opposing edges of a wound
- Tying knots on the barbed section of the material will damage the barbs and potentially reduce the suture tensile strength and barb effectiveness
- For the bidirectional forces to be created and for the device to function properly, both sides of the STRATFIX device must be engaged in the tissue
- Additionally, when completing placement, an additional backstitch or bite of tissue lateral to the end of the incision is required to lock the device in place